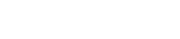
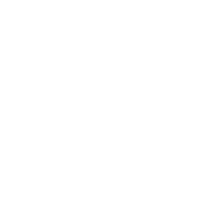
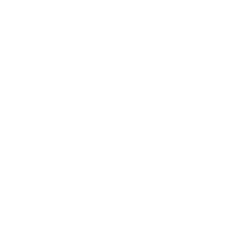T2-weighted images
Methylmalonic acidaemia (MMA) in a child
SectionNeuroradiology
Case TypeClinical Cases
AuthorsEliza Stavride, Katerina Manavi, Melpomeni Kosmidou, Charikleia Mavridou, Filippos Sarafis, Ioannis Tsitouridis
Patient6 years, male
The parents admitted to developmental delay, eating disorders, seizures, iron-deficiency-anaemia under treatment, and surgical excision of adenoids 2 months ago. Workup revealed elevated ammonia and methylmalonyc acid levels and low vitamin-B12 levels in the blood.
The abdomen ultrasound and chest X-ray showed no pathology.
It is caused by complete or partial deficiency of the enzyme methylmalonyl-CoA mutase (mut0 enzymatic subtype or mut– enzymatic subtype, respectively), a defect in the transport or synthesis of its cofactor, adenosyl-cobalamin (cblA, cblB, or cblD-MMA), or deficiency of the enzyme methylmalonyl-CoA epimerase [2]. These enzymes are essential for the body to convert the amino acids isoleucine, valine, methionine and threonine and also the cholesterol to propionic acid, methylmalonic acid and succinic acid.
It is estimated that as many as 60% of cases are the result of a mutated MUT gene encoding the protein methylmalonyl-CoA mutase, which converts methylmalonic-CoA into succinyl-CoA. As a result of lack of this enzyme, methylmalonyl-CoA and other potentially toxic compounds accumulate in the body's organs, blood and tissues, causing the signs and symptoms of MMA [3]. Mutations in the MMAA, MMAB, and MMADHC genes can also hinder the proper function of methylmalonyl-CoA (each of these encodes a protein required for its proper function). Mut0 subtype, where there is complete lack of the enzyme, is the most severe form of MMA and has the poorest outcome. Whereas, mut- subtype, where mutations change the structure of the enzyme without eliminating its activity, is typically less severe and with variable symptoms than the mut0 form [3].
Onset of the manifestations of MMA ranges from the neonatal period (when proteins are added to the infant's diet) to adulthood and vary from mild to life-threatening [2,3,4]. It can present symptoms such as vomiting, lethargy, dehydration, hypothermia, hypotonia, respiratory distress, kidney failure, pancreatitis, seizures, stroke, progressive encephalopathy, severe ketoacidosis, hyperammonaemia, neurtropaenia, thrombocytopenia, and developmental delays [2,3].
Diagnosis is based on the high concentration of methylmalonic acid in urine and blood and can also be indicated through the use of CT and MR imaging of the brain. The most common findings of MMA on imaging is ventricular dilation, cortical atrophy, subcortical white matter abnormality, delay in myelination and abnormalities (T2 high intensity and diffusion restriction) in the basal ganglia, especially in the globi pallidi [5,6].The finding of biallelic pathogenic variants in one of the five genes associated with MMA can establish diagnosis [2].
Management consists of a protein-restricted diet, carnitine and parenteral vitamin B12 [2, 7].
[1] Oberholzer VG, Levin B, Burgess EA, Young WF (1967) Methylmalonic aciduria. An inborn error of metabolism leading to chronic metabolic acidosis. Archives of Disease in Childhood 42: 492-504 (PMID: 6061291)
[2] Manoli I, Sloan JL, Venditti CP (2016) Isolated Methylmalonic Acidemia. Gene Reviews (PMID: 20301409)
[3] Genetics Home Reference (2017) Methylmalonic Acidemia (https://ghr.nlm.nih.gov/condition/methylmalonic-acidemia#sourcesforpage). U.S National Library of Medicine
[4] Mahmud S, Awais UI Hassan Shah S, Ali S (2015) Methylmalonic Acidemia. J Coll Physicians Surg Pak 25(6):462-4 (PMID: 26101005)
[5] Radmanesh A, Zaman T, Ghanaati H, Molaei S, Robertson RL, Zamani AA (2008) Methylmalonic acidemia: brain imaging findings in 52 children and a review of the literature. Pediatr Radiol 38(10): 1054-61 (PMID: 18636250)
[6] Warren Chang, Neilesh Gupta, Dawn Duane, Patrick Barnes, Kristen Yeom (2012) Atypical imaging findings in the setting of methylmalonic acidemia in an infant. Radiology Case Reports Volume 7, Issue 4, Article 749 (PMID: 27330598)
[7] Parayl Sankaran Bindu, Jerry M.E. Kovoor, Rita Christopher (2010) Teaching NeuroImages: MRI in methylmalonic acidemia.
| URL: | https://eurorad.org/case/15037 |
| DOI: | 10.1594/EURORAD/CASE.15037 |
| ISSN: | 1563-4086 |
This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.